Introduction
High-dose methotrexate (HDMTX) is used for the treatment of hematologic malignancies but its use may be limited by delayed methotrexate elimination and acute kidney injury (AKI). Current guidelines for glucarpidase recommend administration between 48-60 hours following the start of a 24-hour HDMTX infusion based on methotrexate concentration at 36-, 42-, or 48-hours [Ramsey 2016]. However, this may be too late to prevent permanent toxicities in cases where significant AKI develops early during infusion. Recent evidence from pig models have shown that early methotrexate measurements can predict the development of AKI [Buddington 2023]. The aim of this study is to determine whether early elimination of methotrexate (soon after completion of the infusion) is a useful “early clearance biomarker” of delayed methotrexate elimination and AKI in humans.
Methods
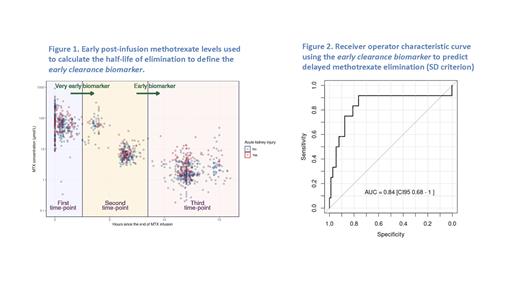
Retrospective data from two sites in Spain that collected two or more early measurements of methotrexate levels from the end of HDMTX infusion were analyzed from a larger HDMTX registry. Methotrexate half-life was calculated employing exponential decay equations and the slope of a line connecting two out of three early time points; each combination was tested, and the best performing pair is reported. Early levels were tagged according to visual exploration and k-means cluster analysis (Figure 1). Delayed methotrexate elimination was defined as a concentration above 1 μmol/L after 42-hours from the start of infusion (micromolar criterion) or above 2 standard deviations (SD) from the population mean modeled using MTXPK.org at 42- or 48-hours from the start of infusion (SD criterion). Receiver operating characteristic (ROC) curves were fitted, with their respective area under the curve (AUC) and confidence intervals for delayed methotrexate elimination and AKI. Additionally, a logistic regression model was employed to predict AKI using a base model with covariates (age, sex, HDMTX dose, and first course) plus early methotrexate clearance.
Results
Data from 144 patients who received 453 HDMTX courses were included. Patient characteristics were as follows: 41% females, age ranged from 1.2 to 81.5 years at the time of HDMTX infusion; diagnoses included acute lymphoblastic leukemia, 79%; non-Hodgkin lymphoma, 21%. Median methotrexate dose was 4,856.9 mg/m 2 [IQR 2,871.7 - 4,985.0], with 88% of the courses infused over 24 hours and 12% over 4 hours. Delayed methotrexate elimination was found in 41% and 6.6% of the courses according to the micromolar criterion and SD criterion, respectively. AKI developed in 29.6% of courses. Median time for the first, second and third measurements were 0.25 [IQR 0.00 - 0.71], 6.32 [IQR 5.86 - 6.69] and 12 [IQR 11.68 - 12.50] hours, respectively. Half-life estimation between the second and third early time-points predicted delayed methotrexate elimination by the micromolar criterion with an ROC AUC of 0.81 [CI95 0.76 - 0.87] or SD criterion with an ROC AUC of 0.84 [CI95 0.68 - 1.00] (Fig. 2) and any grade AKI with an ROC AUC of 0.62 [CI95 0.55 - 0.69]. After adjusting for age, sex, HDMTX dose, and first course, half-life remained statistically significant in the logistic regression model, with an odds ratio of 1.4 [CI95 1.1 - 1.8] (p < 0.01). The addition of half-life to the base model improved the overall performance (ANOVA, p < 0.01).
Conclusions
The half-life of methotrexate elimination soon after completing the HDMTX infusion defines an early clearance biomarker that predicts delayed methotrexate elimination and AKI. It requires only the measurement of 2 early methotrexate levels soon after completing the infusion, and thus represents a practical and applicable method to identify patients at risk who may need pre-emptive interventions like increased hydration or glucarpidase.
Disclosures
Rizzari:CLINIGEN, JAZZ, SERVIER, SERB: Consultancy, Honoraria, Speakers Bureau. Howard:Sanofi: Consultancy, Speakers Bureau; Jazz Pharma: Consultancy, Speakers Bureau; BTG Pharma: Consultancy, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal